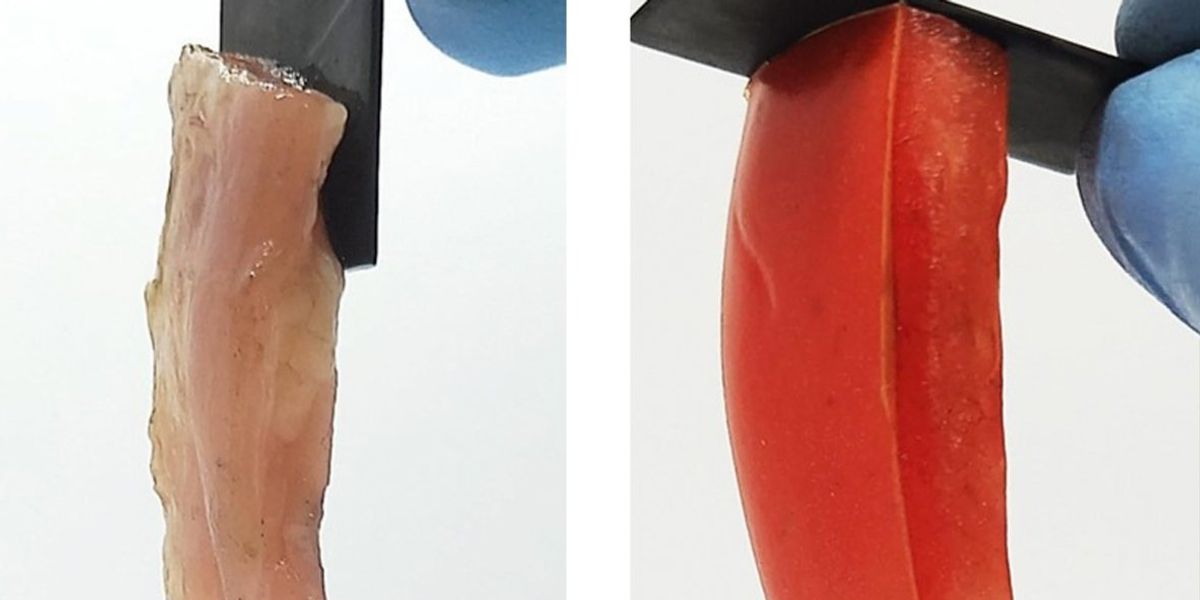
Applying electricity for a few seconds to a soft material, such as a slice of raw tomato or chicken, can strongly bond it to a hard object, such as a graphite slab, without any tape or glue, a new study finds. This unexpected effect is also reversible—switching the direction of the electric current often easily separates the materials, scientists at the University of Maryland say. Potential applications for such “electroadhesion,” which can even work underwater, may include improved biomedical implants and biologically inspired robots.
“It is surprising that this effect was not discovered earlier,” says Srinivasa Raghavan, a professor of chemical and biomolecular engineering at the University of Maryland. “This is a discovery that could have been made pretty much since we’ve had batteries.”
In nature, soft materials such as living tissues are often bonded to hard objects such as bones. Previous research explored chemical ways to accomplish this feat, such as with glues that mimic how mussels stick to rocks and boats. However, these bonds are usually irreversible.
They tried a number of different soft materials, such as tomato, apple, beef, chicken, pork and gelatin…
Previously, Raghavan and his colleagues discovered that electricity could make gels stick to biological tissue, a discovery that might one day lead to gel patches that can help repair wounds. In the new study, instead of bonding two soft materials together, they explored whether electricity could make a soft material stick to a hard object.
The scientists began with a pair of graphite electrodes (consisting of an anode and a cathode) and an acrylamide gel. They applied five volts across the gel for three minutes. Surprisingly, they found the gel strongly bonded onto the graphite anode. Attempts to wrench the gel and electrode apart would typically break the gel, leaving pieces of it on the electrode. The bond could apparently last indefinitely after the voltage was removed, with the researchers keeping samples of gel and electrode stuck together for months.
Howeve, when the researchers switched the polarity of the current, the acrylamide gel detached from the anode. Instead, it adhered onto the other electrode.
Raghavan and his colleagues experimented with this newfound electroadhesion effect a number of different ways. They tried a number of different soft materials, such as tomato, apple, beef, chicken, pork and gelatin, as well as different electrodes, such as copper, lead, tin, nickel, iron, zinc and titanium. They also varied the strength of the voltage and the amount of time it was applied.
The researchers found the amount of salt in the soft material played a strong role in the electroadhesion effect. The salt makes the soft material conductive, and high concentrations of salt could lead gels to adhere to electrodes within seconds.
“It’s surprising how simple this effect is, and how widespread it might be”
The scientists also discovered that metals that are better at giving up their electrons, such as copper, lead and tin, are better at electroadhesion. Conversely, metals that hold onto their electrons strongly, such as nickel, iron, zinc and titanium, fared poorly.
These findings suggest that electroadhesion arises from chemical bonds between the electrode and soft material after they exchange electrons. Depending on the nature of the hard and soft materials, adhesion happened at the anode, cathode, both electrodes, or neither. Boosting the strength of the voltage and the amount of time it was applied typically increased adhesion strength.
“It’s surprising how simple this effect is, and how widespread it might be,” Raghavan says.
Potential applications for electroadhesion may include improving biomedical implants—the ability to bond tissue to steel or titanium could help reinforce implants, the researchers say. Electroadhesion may also help create biologically inspired robots with stiff bone-like skeletons and soft muscle-like elements, they add. They also suggest electroadhesion could lead to new kinds of batteries where soft electrolytes are bonded to hard electrodes, although it’s not clear if such adhesion would make much of a difference to a battery’s performance, Raghavan says.
The researchers also discovered that electroadhesion could occur underwater, which they suggest could open up an even wider range of possible applications for this effect. Typical adhesives do not work underwater, since many cannot spread onto solid surfaces that are submerged in liquids, and even those that can usually only form weak adhesive bonds due to interference from the liquid.
“It’s hard for me to pinpoint one real application for this discovery,” Raghavan says. “It reminds me of the researchers who made the discoveries behind Velcro or Post-it notes—the applications were not obvious to them when the discoveries were made, but the applications did arise over time.”
The scientists detailed their findings online 13 March in the journal ACS Central Science.
The post “Electroadhesion Heralds New Implant And Robot Tech” by Charles Q. Choi was published on 03/19/2024 by spectrum.ieee.org